Abstract
Introduction ROMEI (RUX Observational study in Myelofibrosis treated patiEnts in Italy) is a prospective observational study that aims to bridge the knowledge gap between clinical experience of registration trials and routine patient management by following 500 MF patients (pts) treated with RUX in everyday clinical practice. Enrollment started in April 2017 and closed in May 2022, reaching the target. Herein we describe the RUX dosing pattern that emerged during the observation period for the cohort of pts entering this interim analysis.
Methods An interim analysis was scheduled when all enrolled pts completed the first 12 months of follow-up or prematurely interrupted the study by May 31, 2019.The first visit of the last enrolled patient plus 12 months was established as cut-off date for the analysis.
RUX starting dose overall according to baseline platelet count (<100 x109/L, 100-200 x109/L and >200 x109/L), mean daily dose (mg/day) overall and by initial dose (i.e. 10, 15, 20, 30 or 40 mg/day) were analyzed. Mean daily dose was calculated as cumulative total dose of RUX/ number of treated days in each month (only days in which the patient was effectively treated were considered for the mean daily dose computation). RUX dose changes (number and reasons), interruptions (temporary/permanent and reasons) and time to permanent discontinuation were collected. Patients who withdrew consent from the study or were lost to follow-up were censored at the time of consent withdrawal or last contact date, respectively. Indeed, those still in treatment at the cut-off date for the analysis were censored at the cut-off date.
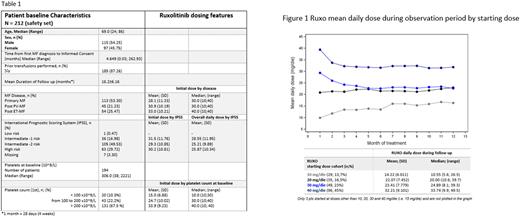
Results 212 pts took at least one dose of study medication (Safety Set) and were evaluable for this analysis. Table 1 shows the most relevant baseline patient characteristics and initial RUX dose. The mean initial RUX dose was equal to 29.9±10.9 mg/day (range 10-40 mg/day). RUX mean daily dose in the overall period of observation was 25.8±10.3 mg/day (range 5.6-49.5 mg/day), slightly higher in the first month and stable with mild fluctuation over time. According to initial RUX dosage, mean daily dosage seemed to decrease by month in pts starting with 30 and 40 mg/day, slightly increase in those starting with 10 mg/day and quite stable in pts starting at 20 mg/day (Fig 1).
Out of the 212 pts, 168 (79%) had at least one change in RUX dosage, and the mean number of changes was 2.4 ± 2.39 (range 0 - 12). The proportion of pts with at least one change in RUX dosage was slightly higher with the increase of IPSS risk: 69%, 78% and 86% in Intermediate-1 risk, Intermediate-2 risk and High risk, respectively.
Fifty-three temporary interruptions were reported by 39 pts (18%). Most of them were due to adverse events, lab or test abnormality (47, 88.68%). After temporary interruption, most pts restarted RUX at same dosage that they took before interruption (62%) while 30% restarted at a lower dosage and only 7.5% at a higher dosage. The first temporary interruption in these 39 pts was observed at a median time of 57 days (range 7-482) from treatment start and the mean duration of interruptions was 21.1±30.9 days (range 1-210 days). As expected, both temporary and permanent discontinuation were higher in pts with platelets < 100 x109/L.
Fifty-five pts (26%) permanently discontinued RUX, and the main reasons (frequency >10%) were adverse events (18%), stem cell transplantation (16%), death (14.5%), physician's decision (13%) and lost to follow-up (11%). The 6-month and the 12-month permanent discontinuation rates estimated were 13.3% (95% CI: 9.4, 18.7) and 21.5% (95% CI: 16.6, 27.8), respectively.
Conclusions RUX dosing pattern emerging from the ROMEI study shows a potential sub-optimal use in clinical practice versus approved indications at the time of data cut-off (May 2019). Approximately 25% of pts in the higher platelet count (>200 x 109/L group) were not assigned to the recommended starting dose (20 mg bid) receiving a lower dosage. Most patients received dose adjustment during treatment while a limited number of patients permanently discontinued RUX, suggesting that dose modulation allows to maintain treatment over time. Additional analysis will elucidate whether the choice of baseline dosage and modulation over treatment might further impact treatment outcome in the short and long term.
Disclosures
Breccia:Novartis, Incyte, Pfizer, BMS, Abbvie: Honoraria. Guglielmelli:Novartis, Abbvie: Other: Other member of advisory board, speaker at meeting. Palumbo:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Siragusa:Csl Behring, Takeda, Amgen, Novartis, Bayer, Sobi, Novo Nordisk: Honoraria. Abruzzese:BMS, Incyte, Novartis, Pfizer: Consultancy. Benevolo:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Crugnola:Novartis: Speakers Bureau; Amgen: Speakers Bureau. Pane:Pfizer: Speakers Bureau; Incyte, BMS, Janssen, Amgen, Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Carli:Novartis: Consultancy. Liberati:Takeda: Research Funding; iQVIA: Research Funding; PSI: Research Funding; Verastem: Research Funding; Secura Bio: Research Funding; Sanofi: Research Funding; Roche: Research Funding; Novartis: Research Funding; Morphosys: Research Funding; Janssen: Research Funding; DR REDDY'S LABORATORIES SPA: Research Funding; Celegene: Research Funding; BMS: Research Funding; Beigene: Research Funding; AbbVie: Research Funding; LOXO: Research Funding; MEI-PHARMA: Research Funding; EPZIME: Research Funding. Binotto:Novartis, BMS, Incyte, Pfizer: Honoraria. De Stefano:GlaxoSmithKline: Honoraria, Speakers Bureau; Bristol Myers Squibb/Celgene: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; AbbVie: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Barberio:Novartis: Current Employment. Misto:Novartis: Current Employment. Passamonti:BMS: Research Funding; Novartis, Celgene, BMS, Abbvie, Janssen, Roche, AOP Orphan, Karyopharma, KYOWA KIRIN, Mei: Consultancy, Honoraria, Speakers Bureau. Palandri:Kartos/Telios: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; CTI: Consultancy, Honoraria; AOP: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.